Research
Research
ATP-fueled protein machines
ATP-fueled protein machines implicated in protein quality control
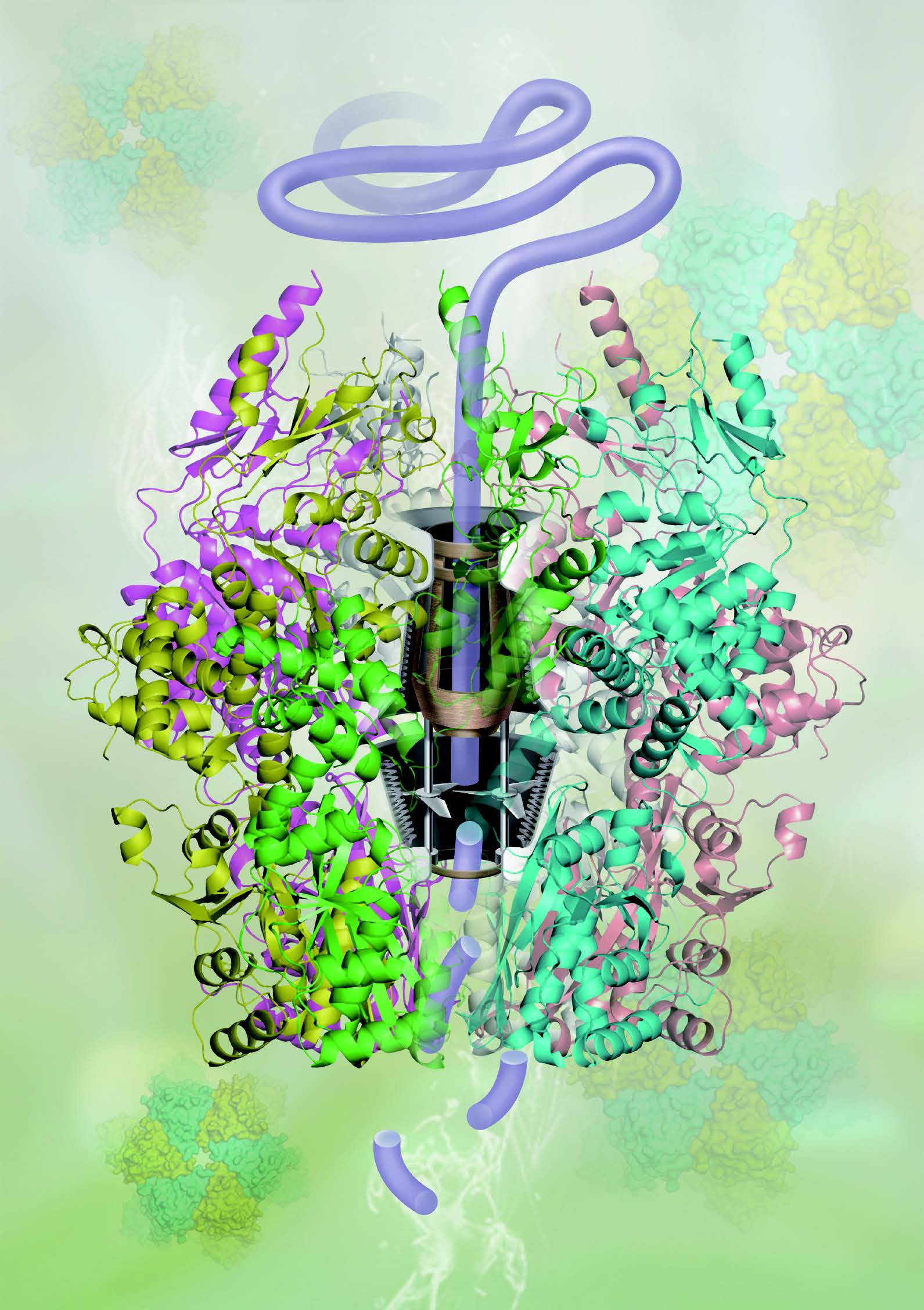
Crystal structure
of Lon protease
ATP-dependent proteases belonging to the AAA+ (ATPases associated with various cellular activities) family play key roles in protein quality control and metabolic regulation by eliminating abnormal/damaged proteins and short-lived regulatory proteins. Lon is the first identified ATP-dependent protease, whose orthologues are distributed in all kingdoms of life including human being. Lon is divided into LonA and LonB subgroups; LonA proteases comprise an N-terminal domain, an ATPase domain, and a protease domain whereas LonB proteases have an ATPase domain with a membrane-anchoring region and a protease domain. We reported the 2.15 Å resolution crystal structure of LonB from Thermococcus onnurineus NA1. Now we are eager to understand the ATP-fueled mechanical motion to drive unfolding and translocation of substrates.
Proteins must be correctly folded to adopt their functional three-dimensional structures, which is essential for the survival of cells. Accumulation of misfolded and denatured proteins is engaged in the progression of several diseases including neurodegeneration and cancer. In cells ATP-fueled chaperonins (CPNs) play critical roles in proper folding of nascent proteins or in refolding of proteins denatured by stresses. CPNs are oligomeric complexes that have a huge chamber surrounded by subunits, and ATP-hydrolysis is coupled with conformational changes of CPNs to encapsulate non-native proteins inside the chamber. We are studying the structure and function of megadalton-sized CPN.
[Related publications]
Young Jun An¶, Sara E. Rowland¶, Jung-Hyun Na¶, Dario Spigolon, Seung Kon Hong, Yeo Joon Yoon, Jung-Hyun Lee, Frank T. Robb*, and Sun-Shin Cha*
"Structural and mechanistic characterization of a novel archaeal-like chaperonin from a thermophilic bacterium"
Nature Communications, Oct 10;8(1):827 (2017)
Young Jun An, Sara E. Rowland, Frank T. Robb, Sun-Shin Cha*
"Purification, crystallization, and preliminary X-ray crystallographic analysis of the Group III chaperonin from Carboxydothermus hydrogenoformans"
Journal of Microbiology, Jun;54(6):440-444 (2016)
Young Jun An, Jung-Hyun Na, Myung-Il Kim, and Sun-Shin Cha*
"Structural basis for the ATP-independent proteolytic activity of LonB proteases and reclassification of their AAA+ modules"
Journal of Microbiology, Oct;53(10):711-717 (2015)
Sun-Shin Cha¶*, Young Jun An¶, Chang Ro Lee, Hyun Sook Lee, Yeon-Gil Kim, Sang Jin Kim, Kae Kyoung Kwon, Jung-Hyun Lee, Michael R. Maurizi*, Sung Gyun Kang*
"Crystal structure of Lon protease: Molecular architecture of gated entry to a sequestered degradation chamber"
The EMBO Journal Oct 20; 29(20):3520-3530 (2010)
Young Jun An, Chang-Ro Lee, Supangat Supangat, Hyun Sook Lee, Jung-Hyun Lee, Sung Gyun Kang, and Sun-Shin Cha*
"Crystallization and preliminary x-ray crystallographic analysis of Lon from Thermococcus onnurineus NA1"
Acta Crystallogr Sect F Struct Biol Cryst Commun Jan 1;66(Pt 1):54-56 (2010)

